the plant immune system
|
Some of the oldest and most impressive organisms on Earth are plants. Being able to thrive over hundreds to thousands of years in environments full of potentially harmful microbes requires a strong immune system. Plants have evolved macroscopic traits to combat environmental and biotic stresses, including thick outer layers of bark and waxy leaf cuticles, prickly spikes such as needles and thorns, and unsavoury chemical profiles that can deter herbivores.
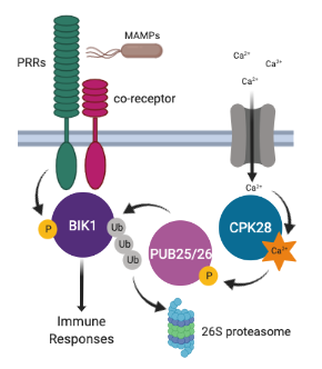
Should a microbe or pest breach one of those barriers, they will be met with microscopic defenses at the plant cell or tissue level. Plant cell walls are made up of sugars such as pectin that form a strong mesh that is difficult for pathogens to penetrate. Microbes that do live within plant tissues mostly colonize areas adjacent to plant cells called the apoplast. If a plant cell recognizes a pathogen it often leads to localized cell death in order to save neighbouring tissues. A full immune response like this is costly and involves release of reactive molecules that can also damage the plant if not kept in check. Plant immune responses are therefore tightly controlled: immunity is turned on only when needed, and turned off as soon as the threat has cleared. |